

The results will be automatically generated. Simply input the accepted (true) value and the observed value and click on the 'Calculate' button. Of course, this depends on the actual reaction sequence and how challenging it is to complete. Percent Error Calculator You can use the percent error calculator to quickly and easily compute the percentage error between the true and observed values of a measurement. Over 90% yield is considered excellent, 80-90% is deemed very good, 70-80% is good, 50-70% is fair, and below 40% is poor. If the darts were thrown again, very close to the. In case of an error, use normal text-editing procedures. For example, if several darts were thrown at a dart board, and all hit the same spot far away from the bull’s-eye, we could say the throws were precise, but inaccurate. Alternately, press the TAB key until the cursor appears in this blank, then type the number. Precision shows how much a repeated measurement will change its value. You might be wondering what is considered a good percent yield value. Accuracy is the quality that a measurement has if it is close to some other quantity’s true value. Therefore, the goal of a chemist working on improving a reaction is most often to improve the yield. You can calculate the error percentage by using our percent error calculatoror the formula below. Since it’s a direct measure of the efficiency of a reaction, it is an important indicator of the amount of reactant required to make the actual amount of product you want. Percent yield is a very important consideration in commercial and industrial chemistry. So we might simplify the concept of percent loss to be 100% minus the percent yield, or basically the remaining reactant that did not react or formed impurities in the experiment. So, in the example above, the percent loss is 7.8%.Ī keen observer might have noticed that the percent yield of 92.2% added to the percent loss of 7.8% equals exactly 100%. Click on the link and use them to save your time.For example, let’s calculate the percent loss in the reaction above with a theoretical yield of 4.85 g and an actual yield of 4.47 g. Get this amazing calculator at and not only this, we have many other statistics calculators. Percent Accuracy = (|(Vo - Vₐ)|/Vₐ) * 100 The percent error formula is the difference between a calculated or measured value and its real or exact value, which is then divided by its said value and.
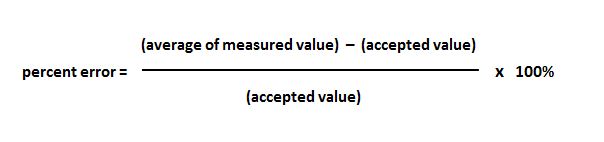
Taylor experimentally finds this liquid to have a. Given values, observed value (Vo)= 92, Accepted value (Vₐ)= 95.6 The Handbook of Chemistry and Physics lists the density of a certain liquid to be 0.7988 g/mL. Question 2 : Measure the temperature is equal to 92☏, and we know that our average temperature is equal to 95.6☏. Experimental Value 5.51 grams Known Value 5. The actual mass of the sample is known to be 5.80 grams. Percentage error is a measurement of the discrepancy between an observed (measured) and a true (expected, accepted, known etc.) value. = (((61.1) * (77))+((80)*(1-77))) = 65.46 % Error Relative Error x 100 Example Error Calculations Let's say a researcher measures the mass of a sample to be 5.51 grams. n=125Īccuracy = ((Sensitivity)* (Prevalence)+((Specificity) * (1-Prevalence))įirst find the values for sensitivity, prevalence and specificity. Question:1 Calculate the Accuracy using the below table.

As, we have three methods to calculate we will see an solved example for each method.


 0 kommentar(er)
0 kommentar(er)
